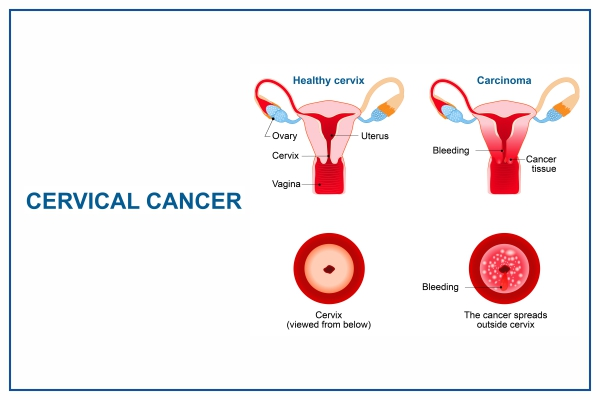
Cervical cancer is a malignancy that originates in the cells of the cervix, the lower part of the uterus connecting to the vagina. It develops slowly from precancerous changes and is primarily caused by persistent infection with high-risk human papillomavirus (HPV) types.
Causes
Persistent HPV infection, especially types 16 and 18, accounts for nearly all cases, transmitted mainly through sexual contact.
Risk factors include multiple sexual partners, early sexual activity, weakened immune system (e.g., HIV), smoking, and long-term oral contraceptive use.
Types
The two main types are
- squamous cell carcinoma (about 90% of cases, from ectocervix cells)
- adenocarcinoma (from glandular endocervix cells)
Rare subtypes include clear cell adenocarcinoma.
Symptoms
Early stages often have no symptoms, but advanced disease may cause abnormal vaginal bleeding (post-intercourse, between periods, or post-menopause), unusual discharge, pelvic pain, or pain during sex.
Diagnosis Methods
Diagnosis involves a Pap smear (cytology) or HPV testing to detect abnormal cells or viruses, followed by colposcopy, biopsy, endocervical curettage, or cone biopsy for confirmation.
Staging uses imaging like CT/MRI or an exam under anesthesia.
WHO prioritizes HPV DNA testing over VIA or cytology (Pap smear) due to its objectivity, higher sensitivity, cost-effectiveness, and ability to prevent more cases.
Self-sampling for HPV testing is endorsed to boost access, especially in low-resource settings.
Screening intervals are 5 to 10 years.
Screening
Regular screening with Pap tests every 3 to 5 years from age 21 to 30 or HPV tests from age 30 detects precancers early; visual inspection with acetic acid (VIA) is used in low-resource settings like India.
Screening and treatment approaches prevent progression.
The latest WHO guidelines on cervical cancer screening stem from the 2021 publication “WHO guidelines for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention,” aligned with the ongoing Global Strategy to accelerate the elimination of cervical cancer, updated through 2025-2026 efforts.
These emphasize high-performance HPV DNA testing as the primary method for equitable, effective screening to meet 90-70-90 targets by 2030:
90% HPV vaccination,
70% screening coverage,
and 90% treatment access.
Screening For General Population
Start at age 30 with high-performance HPV testing; minimum two lifetime screens by ages 35 and 45.
Positive HPV results lead to triage (e.g., VIA, cytology) and screen-and-treat or screen-triage-treat approaches for precancers using thermal ablation, cryotherapy, LEEP, or cone biopsy.
Prevention
HPV vaccination, e.g., Gardasil, for girls 9 to 14 years, prevents most cases; safe sex practices, limiting partners, and avoiding smoking reduce risk.
No routine immunization for boys in many programs, but recommended for comprehensive protection.
Control Measures
Population-based screening programs, vaccination campaigns, early detection via VIA/Pap/HPV tests, and treatment of precancers (cryotherapy, LEEP) are key.
Vector-like surveillance isn’t applicable, but contact tracing for partners aids control.
Treatments
Options depends on stage:
Surgery (cone biopsy, hysterectomy) for early stages;
radiation with/without chemotherapy (cisplatin) for locally advanced;
targeted therapy (bevacizumab) or immunotherapy for metastatic.
Early detection yields high cure rates.
Public Awareness
Campaigns emphasize HPV vaccine acceptance, regular screening benefits, and symptom recognition via mass media, community talks, and digital tools to reduce stigma and myths.
Community Engagement
Involve ASHA workers, self-help groups and camps for door-to-door awareness, free screening drives and vaccination in rural areas, schools, junior colleges, etc.
IEC Materials
Information, education, & communication tools include posters, pamphlets, and videos in local languages on HPV, screening, and vaccines, tailored for low literacy with visuals, e.g., NCVBDC-style modules.
Role of Public Health Department
Departments like India’s NCVBDC, under NHM, lead screening via NPCDCS, vaccine procurement/distribution, training microscopists/health supervisors, surveillance, and IEC; they integrate with RMNCH+A for 90-90-90 targets.
Immunization
HPV vaccines are two doses for girls 9 to 14 years old, with a third dose later, targeting high-risk types; India’s program via school-based/health facilities aims for 90% coverage in girls, with rollout in states like Maharashtra since 2023.
Global Strategy Targets
Achieve 70% of women screened by 35 and 45 with high-performance tests; the model shows this could avert 74 million cases and 62 million deaths by 2120.
Countries track progress via profiles and focus on the continuum of care from screening to treatment.
Types Of HPV Vaccine
HPV vaccines primarily include three main types:
Bivalent (Cervarix, Targeting HPV 16/18 for cancer prevention)
Quadrivalent (Gardasil, covering HPV 6/11/16/18 to also prevent genital warts)
Nonavalent (Gardasil 9, protecting against nine types: 6/11/16/18/31/33/45/52/58 for broadest coverage).
Recommended Schedule
WHO and CDC recommended 2 does (0 and 6-12 months) for ages 9 to 14 (optimal pre-exposure window);
3 doses (0, 1-2, 6 months) for ages 15+ who are immunocompromised, up to 45 years.
India’s NHM program prioritizes girls 9-14 with 2 doses via schools/health centers.
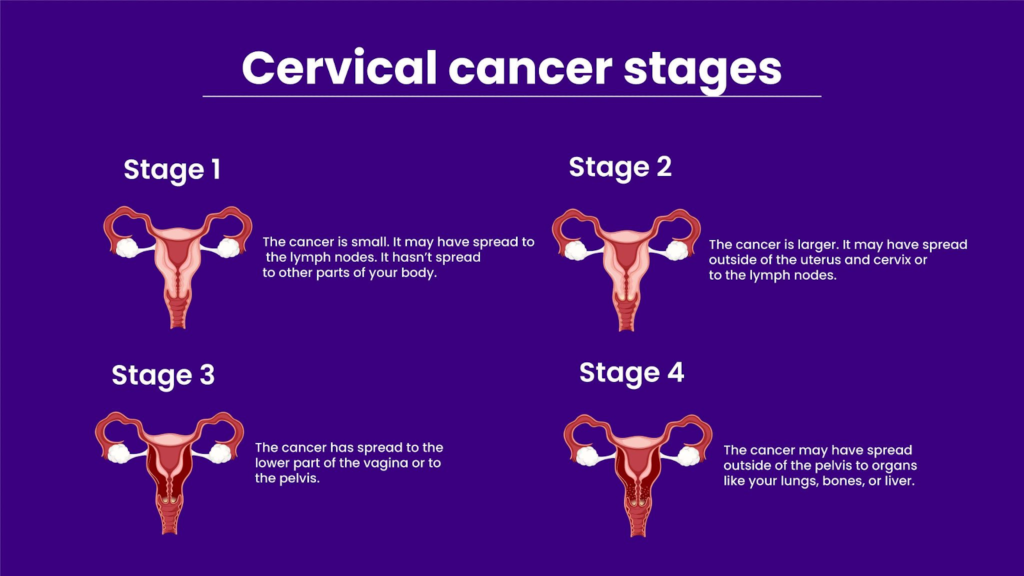
Cervical Cancer Stages
Cervical cancer progresses through stages defined by the FIGO system, from early confined tumors to advanced spread. Diagrams typically show top-down cervix views or uterine cross-sections illustrating tumor growth and invasion.
Stage I: Confined to Cervix
Cancer is only in the cervix, divided by tumor size and invasion depth.
IA1: Microscopic invasion <3 mm deep.
IA2: Microscopic invasion >3 mm but <5 mm deep.
IB1: Visible tumor <2cm.
IB2: Tumor >2cm but <4cm.
IB3: Tumor >4cm.
Illustrations often depict small intraepithelial lesions expanding into stroma.
Stage II: Spread to Upper Vagina or Pelvic Wall
Tumor invades beyond cervix but not to pelvic wall lower third.
IIA: Upper two-thirds of the vagina, no parametrial involvement.
IIB: Parametrium (tissues around uterus).
Cross-section diagrams show vaginal extension and parametrial spread.
Stage III: Lower Vagina or Pelvic Wall
Advanced local spread with possible ureter blockage.
IIIA: Lower vaginal third.
IIIB: Pelvic wall or ureteral obstruction.
IIIC: Lymph node involvement.
Visuals highlight sidewall invasion and node enlargement.
Stage IV: Bladder, Rectum, or Distant Spread
Most advanced with organ invasion or metastasis.
IVA: Bladder/rectum mucosa.
IVB: Distant sites, lungs, liver, bones.
Diagrams illustrate transuterine spread to adjacent organs or metastases. These stages guide treatment from early surgery to chemoradiation.